Cochrane Workshops 2024: Fundamentals of Basic Systematic Review & Network Meta Analysis
|
Programme Duration: |
08 to 12 July 2024 and 22 to 26 July 2024 |
|
Venue: |
Zoom (Online) |
|
Organizer: |
Cochrane Singapore, National University Singapore (NUS) and Singapore Clinical Research Institute (SCRI) |
In today’s fast-paced world of clinical research, where new studies emerge daily, a systematic review serves as a vital tool for making sense of it all.
More than just a method for gathering data, a systematic review offers a rigorous and structured approach to synthesising research evidence on a specific topic or question. By adhering to predefined protocol—with clearly defined criteria for study selection, comprehensive search strategies to identify all relevant research, and standardised quality assessments—it ensures that only the most relevant and credible research is considered.
The strength of a systematic review lies in its ability to minimise bias and provide a transparent, reproducible summary of the evidence. This meticulous process provides a roadmap for the collection and evaluation of data into actionable insights that can inform policy, refine clinical practice, and guide further research.
Cochrane is a global organisation that produces trusted, evidence-based reviews on healthcare interventions. Founded in 1993, it is renowned for its rigorous systematic reviews, which has helped guide healthcare decisions for policymakers, practitioners, and patients worldwide.
In an era driven by evidence-based research, systematic reviews have become an indispensable part of advancing healthcare and science.
In July 2024, the Singapore Clinical Research Institute (SCRI) Health Economics and Outcomes Research (HEOR) team and National University Singapore (NUS) co-organised two virtual workshops, ‘Basic Systematic Review and Meta-Analysis’ and ‘Introduction to Network Meta-Analysis’, designed to impart the knowledge and principles of Cochrane Review. Both workshops were led by trainers from the SCRI HEOR team, who are also representatives of Cochrane Singapore.
Basic Systematic Review and Meta-Analysis Workshop (8 – 12 July 2024)
The Basic Systematic Review and Meta-Analysis Workshop brought together 45 participants from private public healthcare institutes, academic and research organisations. Over the course of five half-days, the workshop featured a blend of lectures and hands-on sessions, providing attendees with the core skills and methodologies required to conduct high-quality systematic reviews.
Participants were introduced to the principles of systematic reviews, from formulating research questions and conducting comprehensive literature searches to assessing risk of bias and extracting data. The workshop also covered advanced topics such as meta-analysis techniques,
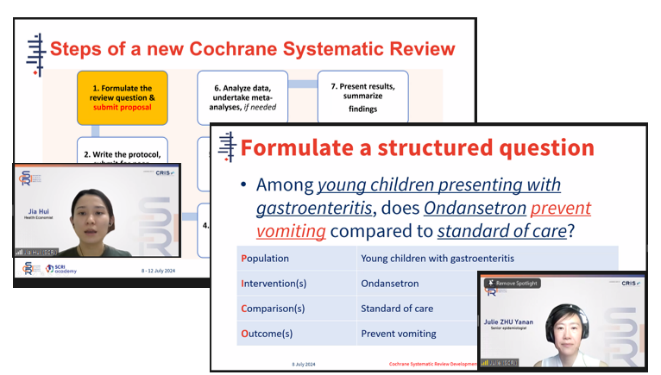
interpreting findings, and the importance of maintaining transparency and reproducibility in research.
SCRI HEOR trainers shared a step-by-step guide on conducting a Cochrane Systematic Review and formulating a structured question using the PICO framework.
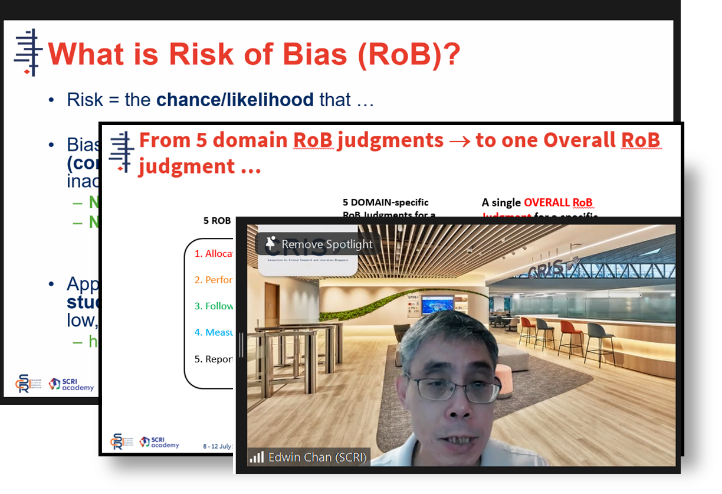
A/Prof Edwin Chan, Chief Scientific Officer of SCRI and Director of Cochrane Singapore, shared about the functionalities of the Cochrane Risk of Bias (Rob) 2.0 tool, the recommended method for assessing bias in randomised trials included in Cochrane Reviews.
Our trainers utilised a combination of theory-based lectures and hands-on sessions, delivering a comprehensive and engaging learning experience. Key topics were introduced through the lectures that laid a strong theoretical foundation, covering crucial concepts such as risk of bias assessment, data extraction, and the creation of Summary of Findings tables.
These lectures were complemented by practical, hands-on sessions where participants had the opportunity to actively apply these principles. Guided by our trainers, they worked on paper exercises and utilised specialised software, translating theory into practice. This blended approach ensured that participants walked away with a deeper understanding of systematic reviews and gained practical skills in conducting them effectively in their own work.

Introduction to Network Meta-Analysis (22 – 26 July 2024)
Building on the foundational principles of systematic reviews and meta-analyses, network meta-analysis (NMA) is an advanced methodological approach that enhances the ability to compare multiple healthcare interventions simultaneously.
While traditional meta-analyses focus on direct comparisons between two interventions, NMA extends this capability by allowing for the comparison of several treatments within a single analysis, even in the absence of direct head-to-head trial data.
By synthesising both direct and indirect evidence across a network of studies, NMA offers a broader and more nuanced understanding of the relative effectiveness of various interventions. The ‘Introduction to Network Meta-Analysis Workshop’ was a dynamic and informative event that brought together local and global researchers and healthcare professionals to deepen their understanding of advanced evidence synthesis techniques. The five half-day workshop was joined by 20 participants, including several who had attended the Basic workshop.
Our trainers delved into the complexities of NMA, covering topics such as statistical models, assumptions, and the interpretation of results. The sessions focused on practical application, with participants engaging in interactive hands-on sessions that involved constructing networks, running analyses, and interpreting the outputs.
The course also spotlighted commonly used software tools in NMA, ensuring participants gained both a strong theoretical foundation and the technical expertise to implement NMAs in their own work.
In previous years, Stata had been the statistical software taught in practical sessions. This year’s workshop featured an additional half-day dedicated to hands-on sessions to familiarise with a new statistical software – RStudio, an open-source tool that offers an alternative to participants.
Participants practised conducting NMA for different data outcomes using Stata and RStudio, respectively. They were also introduced to MetaInsight, a web app designed to allow users to upload data and obtain the NMA results instantly, adding another layer of practical, real-time learning to the experience
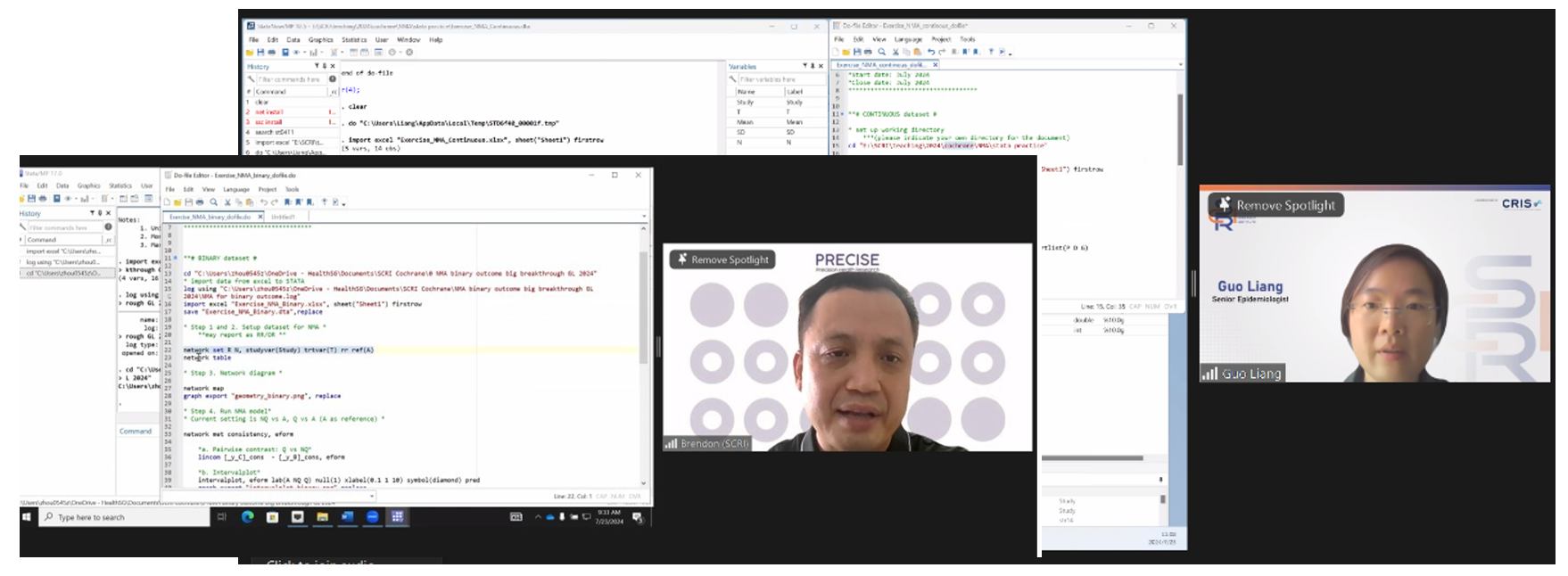
SCRI HEOR trainers sharing concepts and guiding participants during the theory-based and practical sessions.
The workshop concluded on a high note with in-depth sessions on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) and Confidence in Network Meta-Analysis (CINeMA) approaches for assessing the certainty of evidence, and the standards for reporting NMA results.
With an emphasis on the importance of evaluating the certainty of evidence, the final sessions guided participants through the assessment of key factors such as publication bias, indirectness, risk of bias, inconsistency, and imprecision. The concluding discussions spotlighted best practices for reporting NMA results with transparency, ensuring that the conclusions drawn are robust and reliable. By the end of the workshop, participants were equipped with a thorough understanding of how to effectively communicate NMA findings, enhancing the impact of their work on evidence-based healthcare.
The NMA technique has become valuable in complex scenarios where clinicians and principal investigators need to evaluate numerous therapeutic options. With our data-driven healthcare system today, being equipped with these advanced techniques is crucial to stay at the cutting-edge of evidence-based practice and research.
Thank you to all participants for your enthusiasm. We look forward to next year’s intake!
Click here to find out more about Cochrane Singapore.
Click here to learn more about our initiatives.
This event is jointly presented by Cochrane Singapore, Singapore Clinical Research Institute and NUHS Research Support Unit – Systematic Review.

