Clinical Trial Process Enhancement
The Clinical Trial Enhancement Process involves refining and optimizing various aspects of clinical trials to ensure its effectiveness and reliability. This iterative process includes reviewing trial startup processes, trial protocols, participant recruitment strategies, data collection methods, and statistical analysis plans. Enhancement might also focus on minimizing biases, improving patient safety and increasing the trial’s overall efficiency. Regular evaluations and adjustments help trialists and researchers identify potential challenges and implement necessary improvements, ultimately leading to more robust and informative clinical trial outcomes.
Singapore's Clinical Trial Portal - CTSG
CTSG serves as Singapore’s centralised portal for all things related to clinical trials. Crafted to cater to patients, caregivers, clinical investigators and corporations alike, CTSG is dedicated to make clinical trial information more accessible and understandable. By demystifying the clinical trial landscape, CTSG hopes to encourage greater interest in clinical trials, fostering innovation and improving health outcomes. Refer to https://clinicaltrials.sg/ for further information.
Early Phase Community of Practice
In RIE2025, early phase clinical trials have been identified as a focus area for the Singapore clinical trials ecosystem. It was highlighted that this would facilitate building up a cadre of Key Opinion Leaders (KOLs) within the ecosystem, as well as enhance the know-how and capabilities within the three public healthcare clusters to conduct the more complex and sophisticated Phase I & Phase II clinical trials that are crucial for the development of therapeutics and medical devices.
Singapore’s strategic focus on building expertise in early phase clinical trials and creating a favourable environment for clinical research will not only lead to economic benefits, but also contribute to the advancement of medical science and patient care. By fostering a robust early phase clinical trial community of practice, Singapore can establish itself as a leading hub for healthcare, innovative research and pharmaceutical development.
To align with RIE2025 directions, the Singapore Clinical Research Institute (SCRI) will work with the early phase clinical trial units in the three public healthcare clusters to coordinate efforts at a national level so as to avoid duplication of efforts and achieve a level of synergy.
Public Awareness Campaign
Clinical trials are essential for developing new and effective treatments and improving the quality of life for patients. One of the biggest barriers in clinical trial is public awareness. Most people are not aware of what clinical trials involve and why they matter. They are not aware that clinical research can be an accessible and viable treatment options.
In addition, most of the clinical trial information are often written in technical jargon with complicated concepts which patients cannot relate to, and such information are often presented in a way that is difficult to digest for general public. When patients do not understand what their involvement would be, how the trial would be conducted and what are the benefits for participating in clinical trials, it is unlikely that they would sign up for a trial.
As such, it is important to raise public awareness for clinical trials and improve clinical research literacy.
SCRI is working on a public awareness campaign for clinical trials to reach out to public proactively as an national effort.
Primary Objective:
-
To raise awareness and improve the perception on clinical trials among the patients and public by educating them on the rights, benefits and risk when participating in a clinical trial.
Secondary Objective:
-
To aid in increasing subject recruitment rate.
Engaging Clinical Research Ecosystem in Singapore
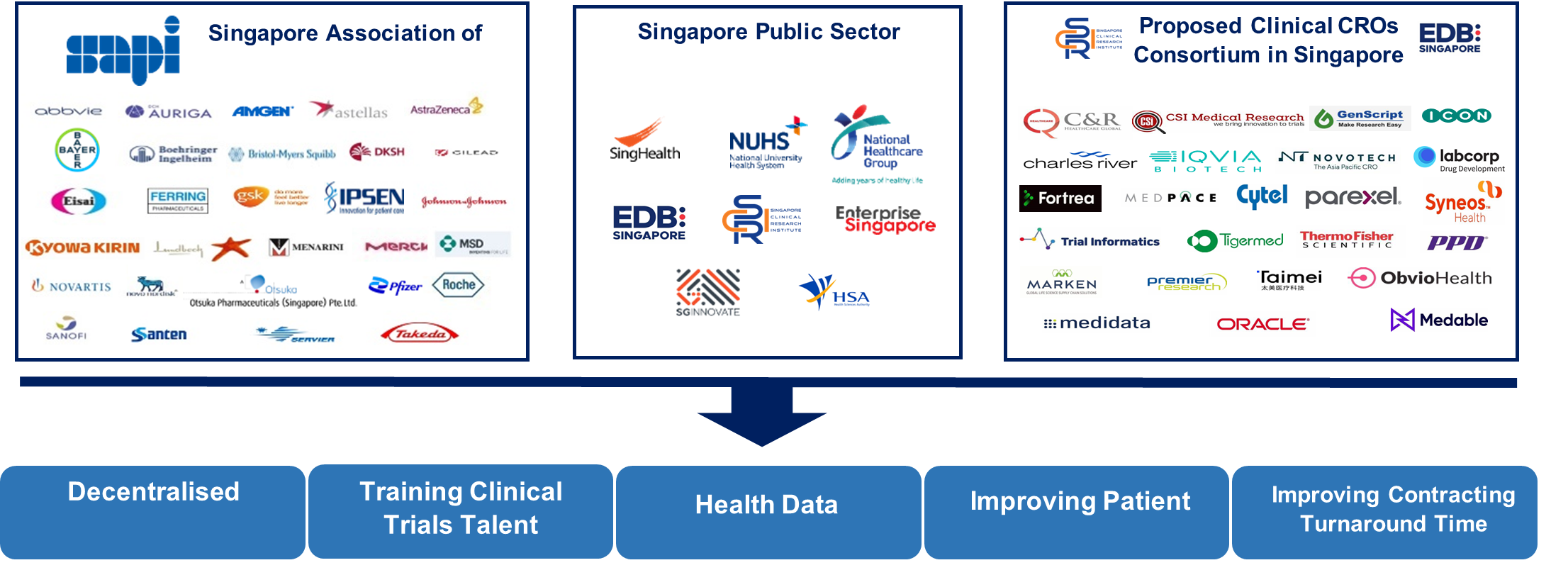
Besides being a national academic research organisation dedicated to enhancing the standards of clinical research in Singapore by developing core capabilities, infrastructure and scientific leadership for clinical research, SCRI is also work closely with the healthcare clusters, government agencies, regulatory bodies, industry partners, CROs and service providers to enhance Singapore’s clinical research ecosystem, foster public-private collaborations as well as provide leadership in the operational and methodological aspects of clinical trials. These efforts have helped Singapore become a more attractive place to private sector investors and will continue to contribute to the enhancement of the local clinical trials landscape in the years to come.

