About SCRI Academy

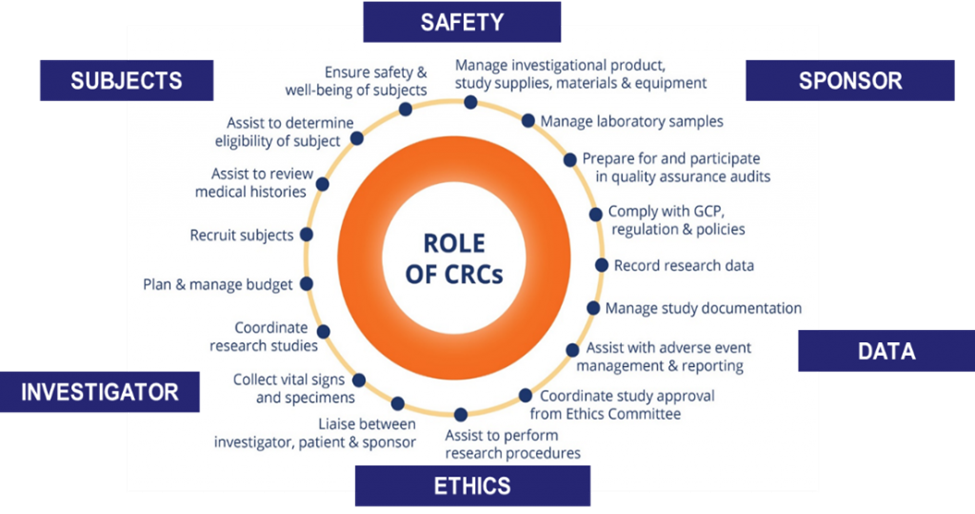
What is a Clinical Research Coordinator?
Clinical research fuels revolutionary progress in medicine, pushing patient care to unparalleled heights of excellence. The linchpin of each successful clinical research study is the Clinical Research Coordinator (CRC), ensuring smooth, ethical and efficient conduct of the studies.
Clinical Research Coordinator (CRC) is a specialized research professional that manages the daily clinical trial activities under the direction of the Principal Investigator (PI). They assist the PI in coordinating and managing the conduct of the clinical trial, developing study materials and tools, recruiting participants, managing data, budget, and ensuring compliance with the relevant policies and regulatory requirements. CRCs also act as liaisons between the study sponsor, PI, and other research staff. They also prepare and maintain study documentation and coordinate communication among stakeholders. Possessing strong organizational and communication skills is essential for CRCs to progress in their careers.

SCRI Academy -National Learning and Development Platform for CRCs
The SCRI Academy was launched on 29 August 2017 by Dr Lam Pin Min, Senior Minister State for Health, to serve as Singapore’s first national learning and development platform for the CRCs.
The aims of the SCRI Academy include:
•Implement the National CRC Learning and Development Initiative
•Form partnerships with local and overseas training providers
•Implement training and certification programmes for clinical research professionals.
Skilled Clinical Research Coordinators play an important role in supporting research investigators in the execution of quality clinical research. Maintaining a well-trained talent pool of CRCs was critical in Singapore’s pursuit to be a leading centre for healthcare innovation, as well as retaining its attractiveness as a site for clinical trials.
In 2017, the Ministry of Health tasked SCRI with the implementation of national training programmes for CRCs and set aside funding for development of CRCs. SCRI consulted the Subject Matter Experts, such as Health Sciences Authority (HSA), and collaborated with various local and overseas education partners to develop a series of structured national CRC training programmes.
The series of structured programmes by SCRI Academy provides a stepwise training for the CRCs to improve on their clinical research knowledge and skills to support their career progression.
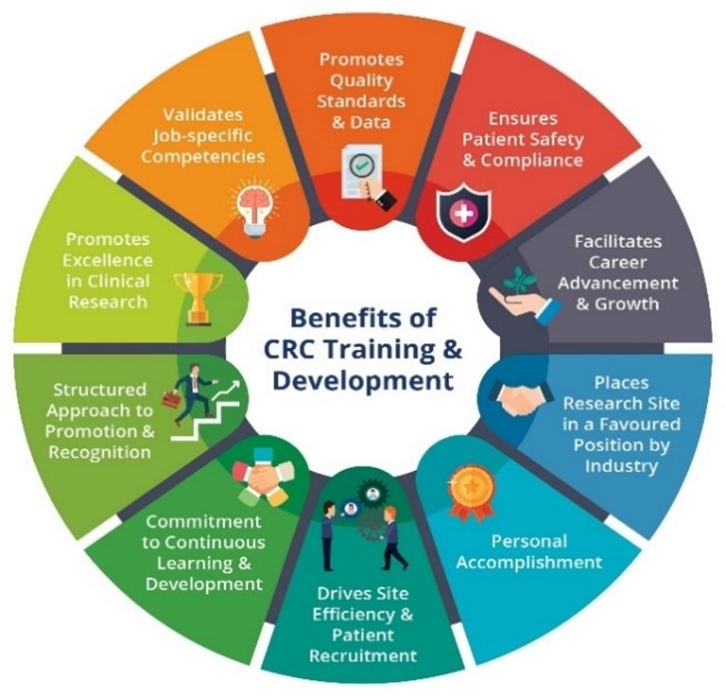
Apart from understanding the requirements, policies and regulations involved in the conduct of a clinical trial, it is also vital for the CRCs to have the ability to apply the theoretical concepts to real-life situations. Therefore, the programmes were designed with comprehensive practice-based activities, case scenarios, demonstrations and classroom discussions to reinforce the application work.

CRC Training by SCRI
I want to try if CRC role fits me
CRC Internship Programme

Experience a comprehensive 6-month internship program at the public healthcare institutions to immerse yourself in the CRC role firsthand. In addition to gaining a deeper understanding of CRC responsibilities, you’ll acquire valuable insights into clinical trial conduct.
Click here to find out more.
I am a CRC
CRC Onboarding Programme

In complementary to the public healthcare institution onboarding programme, SCRI offers a coaching programme tailored for newly-hired CRCs with less than one year of experience. This programme, comprising of 2 half-day sessions, is designed to acquaint CRCs with their roles and responsibilities, providing essential theoretical knowledge and practical tips to navigate basic duties smoothly.
If you are a newly hired CRC employed by the Singapore public healthcare institution and interested to join this programme, click here for more details.
National Training and Certification Programme
-
CRC Level 1 Programme
A 7-day training course that offers a comprehensive introduction to the on-site operations of clinical trials from protocol feasibility to study start-up, ethics and regulation, recruitment and study closure. It is targeted at CRCs with at least a year of experience.
Click here for more details.

-
CRC Level 2 Programme
A 6-day training course that empowers CRCs with project management skills and the ability to coordinate investigator-initiated clinical research studies with reasonable degree of proficiency across a range of study designs. It is targeted at Senior CRCs or equivalent with at least 4 years of experience in coordinating clinical research studies and performing subject recruitment, informed consent and/or subject follow-up.
Click here for more details.
-
CRC Level 3 Programme
An advance 4-day training course designed to develop the essential capabilities critical to the role of a Senior CRC in supporting the management in planning the departmental activities and managing a clinical research team. The programme covers the key operational, leadership and technical elements related to clinical research operation. It is targeted at Senior CRCs and above with at least 8 years of experience in coordinating clinical research studies, who are also involved in conducting quality compliance check and developing study documents and workflow.
Click here for more details.
CRC Alumni
All CRC who have graduated from the National Training and Certification Programme will be offered lifetime mentorship and upskilling opportunities to support their professional development.
CRC Lunch Workshop

To ensure the CRCs stay abreast of the latest updates and trends in clinical research ecosystem, annual lunch workshop is organised. It also offers the CRCs from different public healthcare institutes to mingle, network and delve into industry best practices, fostering deeper insights and connections within the field.
Distinguished Contributor Award for CRCs
The Distinguished Contributor Award for CRCs is given by SCRI Academy to recognise the contributions of outstanding CRCs and their significant contributions to the profession and community in Singapore. The award includes a professional development grant to enable the CRC awardee to further enhance his/her skillsets as a CRC. Click here for more details.
CRC Educator Network
To strengthen the development of the CRC talent pipeline, the CRC Educator Group has been established to nurture clinical research professionals who are passionate in training and developing the next generation of CRCs ensuring a continuity of talent support in the ecosystem.
If you are an experienced CRC who is passionate in training and developing the next generation of CRCs, contact us at scriacademy@scri.cris.sg
I am not a CRC but want to learn more about clinical trial
The Essentials of Clinical Trials for Beginners (E-learning)
A 16-week self-paced course that will chart a roadmap for those who are keen to gain fundamental knowledge on clinical trials. The course covers all the basic knowledge required of a CRC to conduct a trial at site. Click herefor more details.

The Essentials of Clinical Trials for Investigators (E-Learning)
A 16-week self-paced course that will equip a clinician investigator with the essential skills in navigating through the clinical trial activities with confidence and ease. Click here for more details.

How to become a CRC?
Qualifications/Requirements
While specific qualification may vary depending on the institution or organization, a minimum educational requirement for most CRC position is a Diploma or a Bachelor’s degree in a relevant field such as Nursing, Pharmacy or life sciences equivalent.
Progressing in this profession requires strong attention to detail, the ability to manage multiple priorities effectively, and excellent organizational and communication skills. Additionally, self-motivation and aptitude for independent learning are also essential.
Tips for creating an Effective CRC Resume
Creating a well-crafted CRC resume is crucial for standing out in the competitive field of clinical research. Here are some tips to ensure your resume highlights your strengths and experiences effectively:
-
Professional Summary: Start with a concise summary that encapsulates your expertise, key achievements, and career objectives.
-
Highlight Relevant Experience: Emphasize your experience in clinical research, including specific projects, studies, or trials that you have worked on. Detail your role and contributions to these projects.
-
Showcase Key Skills: List relevant skills such as data management, patient recruitment, regulatory compliance, and familiarity with clinical trial software. Highlight any specialized training or certifications.
-
Quantify Achievements: Use metrics to demonstrate your impact. For example, it includes but not limited to the number of trials managed, patient recruited, or amount of research funding secured.
-
Technical Proficiency: Include any specific software or tools you are proficient in such as EDC systems, CTMS, or other clinical research applications.
-
Attention to Detail: Ensure your resume is free from errors, well-organized and tailored to the job you are applying.
How do I get started?
A CRC can work in various places such as hospitals, Clinical Research Organisation (CROs), Pharmaceutical and Biotechnology Companies, and Academic Research Centre.
If you’re ready to take your next steps towards a career as a CRC, here is a list of public healthcare institutions career page to kick-start your journey:
•SingHealth: https://careers.singhealth.com.sg
•NUHS: https://www.nuhscareers.edu.sg/
•NHG: https://careers.nhg.com.sg/NHGHQ
Alternatively, you may consider starting with the short courses on clinical trial to gauge whether clinical research aligns with your interests and aspirations.
The Essential of Clinical Trials for Beginners
The Essentials of Clinical Trials for Investigators
Is the above information useful?
Please scan the QR code below or click here to help us understand your needs better.


